Behavior of Gasses
Introduction:
- The behavior of gasses is a very broad and vast topic that covers a multitude of sub topics. Under this subject, the state of Pennsylvania has picked three main ideas that they require students to know. These three things are Boyle's law, Charle's law, and the ideal gas law. This blog post has an explanation of the three, a bit about their history, and how they will be used in the PSSA testing.
Boyle's Law:
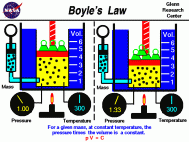
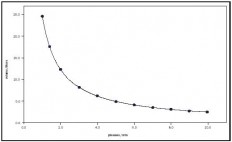
On the PSSA this type of material could be expressed in a question asking for one to solve for one of the variables in the equation. For example, a question could state the starting volume and pressure as well as the final amount of pressure and ask for you to solve for the final volume. Ex:
1. If the pressure of an ideal gas is changed from 145 atm to 290 atm, what is the gasses change in volume, assuming the original volume was 75.0mL?
a. 150 mL
b. 37.5 mL
c.72.5
d. Not enough information is given
Here is a link to an animation of this: http://www.grc.nasa.gov/WWW/k-12/airplane/boyle.html
Charle's Law
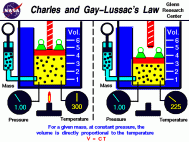
 On the PSSA this type of material could be expressed in a question asking for one to solve for one of the variables in the equation. For example, a question could state the starting volume and temperature as well as the final temperature and ask for you to solve for the final volume. Ex:
On the PSSA this type of material could be expressed in a question asking for one to solve for one of the variables in the equation. For example, a question could state the starting volume and temperature as well as the final temperature and ask for you to solve for the final volume. Ex:
2. Bob wants to find the volume of a gas in a cylinder. He knows that at a temperature of 30˚C the gas had a volume of 6.0 L. The temperature of the gas is now 35˚C. What is the volume of the gas now? **Note you must convert the temperature from Celsius to Kelvin by adding 273 to the Celsius measure**
a. 7.0 L
b. 175 L
c. 6.09 L
d. 15 L
Here is a link to an animation of this: http://www.grc.nasa.gov/WWW/k-12/airplane/glussac.html
Ideal Gas Equation


On the PSSA this type of material could be expressed in a question asking for one to solve for one of the variables in the equation. Ex.
3. A gas exerts a pressure of .0892 atm in a 5.0 L container at 15˚C. What is the molecular mass of the gas?
a. 0.191 mol
b. 5 mol
c. 0.56 mol
d. 19 mol
Sources:
http://www.shodor.org/unchem/advanced/gas/
http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/idegas.html
http://en.wikipedia.org/wiki/Ideal_gas_law
http://www.metric-conversions.org/temperature/celsius-to-kelvin.htm
http://www.chm.davidson.edu/vce/gaslaws/charleslaw.html
http://www.chm.davidson.edu/vce/gaslaws/boyleslaw.html
http://en.wikipedia.org/wiki/Boyle's_law
http://www.grc.nasa.gov/WWW/k-12/airplane/eqstat.html
http://www.grc.nasa.gov/WWW/k-12/airplane/glussac.html
http://www.grc.nasa.gov/WWW/k-12/airplane/boyle.html
- The behavior of gasses is a very broad and vast topic that covers a multitude of sub topics. Under this subject, the state of Pennsylvania has picked three main ideas that they require students to know. These three things are Boyle's law, Charle's law, and the ideal gas law. This blog post has an explanation of the three, a bit about their history, and how they will be used in the PSSA testing.
Boyle's Law:
- Boyle's law explains the relationship between the volume of a gas and its pressure. It states that as the pressure of a gas increases the volume decreases and in the same manner that as the volume of a gas increases the pressure decreases (assuming that the temperature of the gas is constant). This relationship can be expressed as PV=k, where "p" represents the pressure of the system, "v" represents the volume of the gas, and "k" represents a constant. Robert Boyle, a chemist and physicist, discovered this in the mid 1600's and published his findings in 1662. Boyle executed a series of experiments consisting of confining a fixed amount of gas into a curved glass. He then added different amounts of mercury to the gas sample in order to change its pressure. These findings were then related to this equation: P1V1 = P2V2, which expresses "the before and after volumes and pressures of a fixed amount of gas" (wikipedia). **Note** This equation is the one that will be used on the PSSA test.
On the PSSA this type of material could be expressed in a question asking for one to solve for one of the variables in the equation. For example, a question could state the starting volume and pressure as well as the final amount of pressure and ask for you to solve for the final volume. Ex:
1. If the pressure of an ideal gas is changed from 145 atm to 290 atm, what is the gasses change in volume, assuming the original volume was 75.0mL?
a. 150 mL
b. 37.5 mL
c.72.5
d. Not enough information is given
A link to the answers is provided later.
Here is a link to an animation of this: http://www.grc.nasa.gov/WWW/k-12/airplane/boyle.html
Charle's Law
- Charle's law explains the relationship between the temperature of a gas and its volume. It states that as the temperature of a gas increases the volume of that gas will as well (assuming that the pressure of the gas remains constant). Another thing to note is that they do this proportionately meaning that they increase and decrease at the same scale factor. This relationship was found in a similar manner that Boyle's law was found in. Jacques Charles was the scientist that made this discovery and he did so by confining a gas in a curved class tube and changing its temperature while keeping its pressure constant. The relationship that he discovered can be expressed as V1/T1 = V2/T2 where "V" represents the volume of the gas and "T" represents the temperature of the gas. **Note** This equation is the one that will be used on the PSSA test.
2. Bob wants to find the volume of a gas in a cylinder. He knows that at a temperature of 30˚C the gas had a volume of 6.0 L. The temperature of the gas is now 35˚C. What is the volume of the gas now? **Note you must convert the temperature from Celsius to Kelvin by adding 273 to the Celsius measure**
a. 7.0 L
b. 175 L
c. 6.09 L
d. 15 L
A link to the answers is provided later.
Here is a link to an animation of this: http://www.grc.nasa.gov/WWW/k-12/airplane/glussac.html
Ideal Gas Equation
- The ideal gas equation is a combination of Boyle's law, Charles's law and Avogadro's law. This law can be an approximation of the state and behavior of a gas. The ideal gas law was not discovered and no experiments were directly done to find out this law, but the relationships expressed within this law are a compilation of three laws. This relationship can be expressed through the following equation: PV = nRT equation where "P" represents the pressure of the gas, "V" represents the volume of the gas, "n" represents the number of moles of gas, "R" represents the ideal gas constant, and "T" represents the temperature of the gas. The ideal gas constant is approximately 8.3145 J and can be used to plug into this equation. Another thing to not is that if any two of the variables in the equation are kept constant, the relationship between the other two can be observed.
On the PSSA this type of material could be expressed in a question asking for one to solve for one of the variables in the equation. Ex.
a. 0.191 mol
b. 5 mol
c. 0.56 mol
d. 19 mol
Link to answers:
https://docs.google.com/document/d/1R5SF7QKNLUxS0rOfAdHVabXKqcGsOg7hAcP9tn1n9Pw/edit
Sources:
http://www.shodor.org/unchem/advanced/gas/
http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/idegas.html
http://en.wikipedia.org/wiki/Ideal_gas_law
http://www.metric-conversions.org/temperature/celsius-to-kelvin.htm
http://www.chm.davidson.edu/vce/gaslaws/charleslaw.html
http://www.chm.davidson.edu/vce/gaslaws/boyleslaw.html
http://en.wikipedia.org/wiki/Boyle's_law
http://www.grc.nasa.gov/WWW/k-12/airplane/eqstat.html
http://www.grc.nasa.gov/WWW/k-12/airplane/glussac.html
http://www.grc.nasa.gov/WWW/k-12/airplane/boyle.html

Comments
No comments have been posted yet.
Log in to post a comment.